Bangalore-based 3D printing company Next Big Innovation Labs (NBIL), with eight years bioprinting experience, is leading technology applications, especially in regenerative medicine. It advocates customized bioprinting solutions to address global healthcare challenges effectively.
3D Printing Industry spoke with Piyush Padmanabhan, CEO, Co-Founder, and Director of NBIL to dive deep into the company’s journey and vision in bioprinting.
“The ever-widening gap in transplantable organ demand and availability is something that regenerative tissue engineering is yearning to address. With many technologies coming to the fore to provide solutions to the global organ shortage crisis, the breakthroughs achieved in bioprinting over the last decade has been unparalleled. NBIL champions custom bioprinting as the solution to diverse healthcare challenges. Trivima bioprinters, tailored for wound healing, personalized medications, cancer studies, and patient-specific bone implants, offer a unique platform for creative solutions, supported by constant technology and bioprinting expertise,” said Padmanabhan.

Trivima bioprinter series: From evolution to collaborative ecosystem
Padmanabhan explains that the Trivima bioprinters originated to establish an efficient engineering ecosystem at NBIL. Developed in July 2017, the first Trivima bioprinter, underwent technology validation and was initially utilized for internal projects. Following successful validations, the technology found application at Merck KGaA in Darmstadt, Germany, marking the genesis of a focus on customized engineering, requiring precise adjustments to extrusion, movement, and temperature control systems to meet specific research needs.
In response to global bans on animal testing, Indian researchers turned to customized bioprinting solutions for diverse tissue models. Post-pandemic, this demand expanded to include new-age biomaterials, personalized therapies, and clinical applications of bioprinted tissues. This surge inspired the development of three Trivima bioprinter variants. Trivima Basic enables researchers and clinicians to initiate their bioprinting journey, from biomaterial development to the printing of bioceramic constructs.
Trivima Advanced extends the capabilities of bioprinting, offering researchers and clinicians a broader range of applications. Addressing a crucial need for precise scaffold formation and controlled cell layering, Trivima Pro Bioprinter represents a significant milestone. With its advanced features, including controlled droplet printing (down to 0.5 nL) and pellet-based extrusion, users can create complex scaffolds. These three variants cater to users with diverse needs, from basic applications to those requiring a deeper exploration of the multifaceted world of bioprinting.

“We’re looking to provide a holistic support ecosystem to every user of Trivima Bioprinters. Right from technical support to collaborations. For a niche ecosystem, such as bioprinting, to thrive and find its calling in clinical applications, collaborations are going to be a key driver. To achieve this, enabling the ecosystem and supporting interesting projects that have a true outcome for the community is something we strive to do. Building a strong collaborative ecosystem is ingrained in our DNA and in our efforts to do so, we have partnered with some of the pioneers in the domain,” explained Padmanabhan.
Strategic partnerships with top Indian Institutes
Trivima bioprinters are now being used by some of the top institutes in India such as Central Leather Research Institute (CLRI), Manipal University (Manipal), AIIMS (New Delhi), and IIT Hyderabad. The next step in this journey is the collaboration between NBIL and HiMedia, which is focused on building a strong holistic solution for bioprinting applications such as tissue engineering, cancer biology, personalized medicine, and microfluidics, according to Padmanabhan.
“Our research pans from the technology used in our Trivima Bioprinters to the inclusion of AI/ML algorithms within our bioprinters to the establishment of key partnerships to enhance the prowess of the bioprinting domain. The goal is to build a myriad of technologies that can cater to a wide array of problem statements in the pharmaceutical, cosmetic and clinical domains. Below are some of the technologies and partnerships that we have established towards our goals,” added the CEO.
In November 2018, NBIL got accepted into Merck KGaA’s accelerator program, gaining a unique chance to engage with diverse stakeholders in the pharmaceutical industry. A pivotal project involved developing a multi-well bioprinting system for 96-well and 384-well plates, significantly reducing print time from 16 to under 4 minutes. This enhancement not only improved cell viability in biomaterials but also facilitated the creation of co-culture models crucial for pharmaceutical research.
NBIL’s core focus on minimizing animal testing extends to cosmetics, collaborating with industry and non-profit entities. A significant project involved bioprinting the human epidermis, providing an alternative to animal testing. Unique biomaterials were developed, allowing the printing of skin cells to mimic the top two layers of human skin. The technologies evolved in this project now form integral components of Trivima bioprinters, offered to customers seeking alternatives to traditional animal tests for biomaterials and skin models, according to the CEO.
“Technology, be it in the form of engineering or software, is the key driver when it comes to solutions for complex solutions. Another key factor here is the integration of various stakeholders to ensure the translation of the technology is conducted effectively,” said Padmanabhan. “NBIL actively engages in three focal areas: developing customized bioprinting solutions, collaborating with Microsoft to integrate AI/ML solutions for enhanced Trivima Bioprinter outputs, and working towards a clinically deployable solution for surgery rooms. Regulatory approvals for this medical device are a current focus. Key collaborations with AIIMS, New Delhi, and IIT, Hyderabad, highlight our commitment to clinically translatable bioprinting technology.”
According to Padmanabhan, Dr. Falguni Pati’s team at IIT Hyderabad achieved breakthroughs in bioprinting, notably with the bioprinted cornea project. Their focus on developing advanced biomaterials, complex tissue models, and clinical applications has “spurred motivation.” Trivima Bioprinters found significant applications in Dr. Pati’s lab, marking the beginning of a promising collaboration. At AIIMS, New Delhi, Dr. Sujata Mohanty’s team in the Stem Cell Facility explores novel biomaterials and clinical solutions, inspiring advancements in bioprinting and regenerative medicine across diverse societal strata.
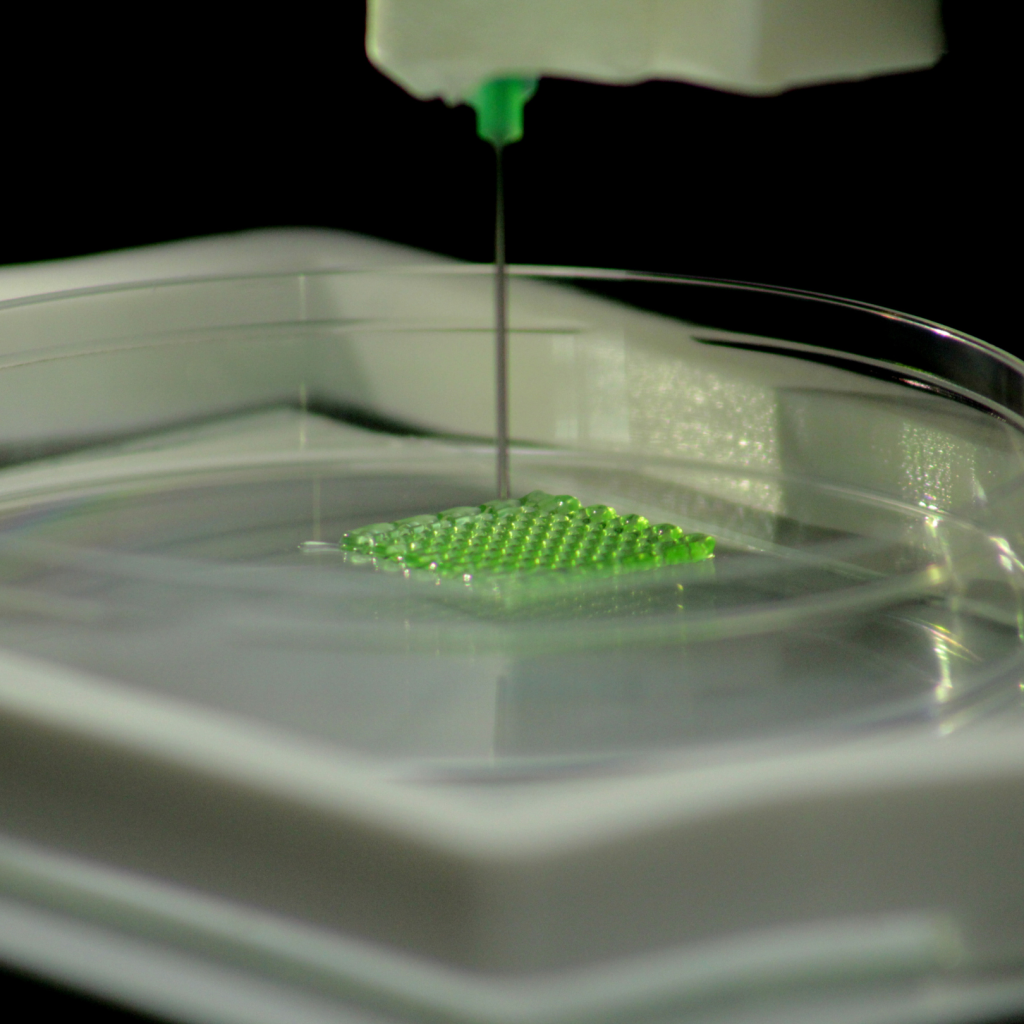
Bioprinting’s progress and hurdles in organ regeneration
Padmanabhan says bioprinting’s applications are extensive, offering promising avenues for addressing organ shortages and reducing rejection risks in transplants. However, realizing fully functional bioprinted organs remains challenging. In tissue engineering, bioprinting constructs scaffolds mimicking the natural architecture of bone tissue. These scaffolds support osteoblast and bone-forming cell growth, facilitating the regeneration of damaged or lost bone. Despite the vast potential, bioprinting continues to evolve, with ongoing exploration in diverse areas of medicine and tissue regeneration.
Additionally, bioprinting is crucial in creating skin constructs for grafts, especially aiding burn victims and those with severe skin injuries. These constructs replicate natural skin by incorporating layers of keratinocytes, fibroblasts, and other components. In cartilage regeneration, bioprinting facilitates scaffold creation by printing layers of bioinks with chondrocytes and supporting biomaterials, replicating the intricate structure of cartilage tissue.
“From a regulatory perspective, we still have a long way to go. What category of regulations bioprinted products are going to fall into is the question! Irrespective of how bioprinters are classified, a notable challenge arises from the fact that bioprinted structures frequently take the form of combination products. These products typically involve cells, biomaterial scaffolds, and/or growth factors. Thus these bioprinted products are likely to span multiple product categories, each of which can be subjected to clearly different regulatory pathways,” said Padmanabhan.
Bioprinting extends to crafting corneal constructs for transplantation and treating corneal diseases. Ongoing research explores bioprinting applications in generating cardiac tissue constructs, including functional heart patches for post-myocardial infarction repair. This evolving landscape signifies the early stages of a promising field, hinting at potential breakthroughs in bioprinting applications on the horizon.
An aim to foster global collaboration for clinical biofabrication advancements
Looking forward, NBIL aims to establish a global biofabrication ecosystem, fostering collaboration among researchers, clinicians, regulators, government entities, and private firms. The focus is on advancing clinical applications through a collective approach and access to breakthrough technologies. According to him, effective cross-talk among stakeholders is deemed essential to realize the practical applications of biofabrication in the clinical realm.
While NBIL has excelled in developing custom bioprinting technology, the focus is now on collaborative efforts with all stakeholders to actualize 3D bioprinted organs. “The challenges we foresee are in the realm of strong regulatory guidelines for bioprinted organs, ensuring a healthy ecosystem for all the stakeholders within the ecosystem to effectively and openly share their ideas, more precision in the technology to print the finer capillaries, heterogenous cell culture and acclimatization of organs before transplantation into the patient,” concluded Padmanabhan.
Read all the 3D Printing Industry coverage from Formnext 2023.
What does the future of 3D printing for the next ten years hold?
What engineering challenges will need to be tackled in the additive manufacturing sector in the coming decade?
To stay up to date with the latest 3D printing news, don’t forget to subscribe to the 3D Printing Industry newsletter or follow us on Twitter, or like our page on Facebook.
While you’re here, why not subscribe to our Youtube channel? Featuring discussion, debriefs, video shorts, and webinar replays.
Are you looking for a job in the additive manufacturing industry? Visit 3D Printing Jobs for a selection of roles in the industry.
Featured image shows NBIL’s team photo. Photo via NBIL.
منبع: https://3dprintingindustry.com/news/interview-nbils-vision-to-3d-print-the-future-of-medicine-one-cell-at-a-time-227069/?utm_source=rss&utm_medium=rss&utm_campaign=interview-nbils-vision-to-3d-print-the-future-of-medicine-one-cell-at-a-time